Unique Device Identification (UDI)
Global Services
Home / UDI Labeling
UDI Labeling
On September 24, 2013, the United States Food and Drug Administration (FDA) released a final rule requiring that most medical devices distributed in the United States carry Unique Device Identifiers (UDI). The UDI system facilitates medical device identification, traceability, and tracking through distribution and use. The rule requires that product information pertaining to the devices be submitted to the FDA’s Global Unique Device Identification Database (GUDID).
In addition, the rule also requires that a new, standardized date format be placed on all medical device labels.
Masimo has updated our labeling for compliance with the UDI regulation and GS1 Standards.
If you have questions beyond the scope of these FAQs, please contact Customer Service:
US: customerorders@masimo.com
Outside US: emeasales@masimo.com
Frequently Asked Questions
Frequently Asked Questions
1. What is UDI?
A Unique Device Identifier (UDI) is a series of numeric or alphanumeric characters based on a global coding standard that adequately identifies a device at the point of distribution and at the point of use. A UDI is composed of:
- A device identifier, the GTIN (Global Trade Item Number): a mandatory, fixed portion of a UDI identifying the specific version or device model and the labeler of that device; and
- A production identifier: a conditional, variable portion of a UDI that identifies one or more of the following when included on the label of the device:
- The production lot or batch within which a device was manufactured;
- The serial number of a specific device;
- The expiration date of a specific device; and/or
- The date a specific device was manufactured.
Masimo uses GS1 as their accredited agency for assigning barcodes and barcode structures.
Please note that any product that is NOT classified as a medical device is not required to contain a barcode.
2. Who has to comply with the UDI regulations?
All medical device companies must comply with the UDI regulations.
3. What is the implementation timeline for complying with the UDI regulations?
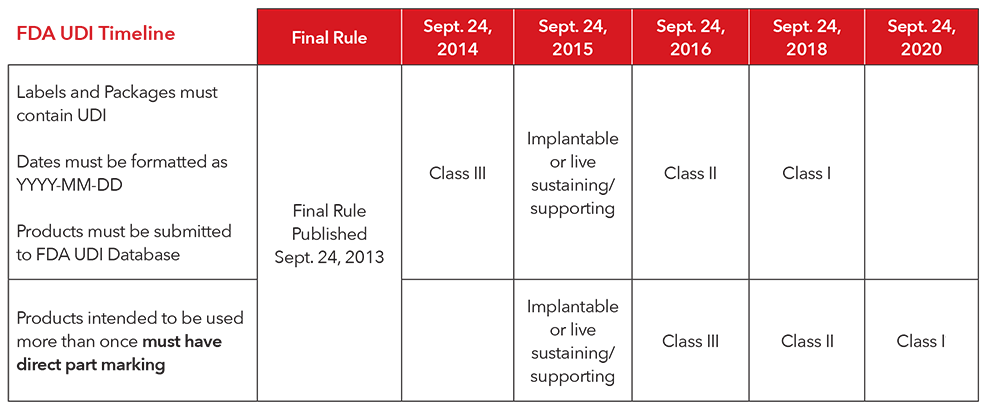
4. How does Masimo comply with UDI?
Masimo distributes Class II and a few Class I products. Masimo has updated our labeling for compliance with the UDI regulation and GS1 standards.
Please note that any product that is NOT classified as a medical device is not required to contain a barcode.
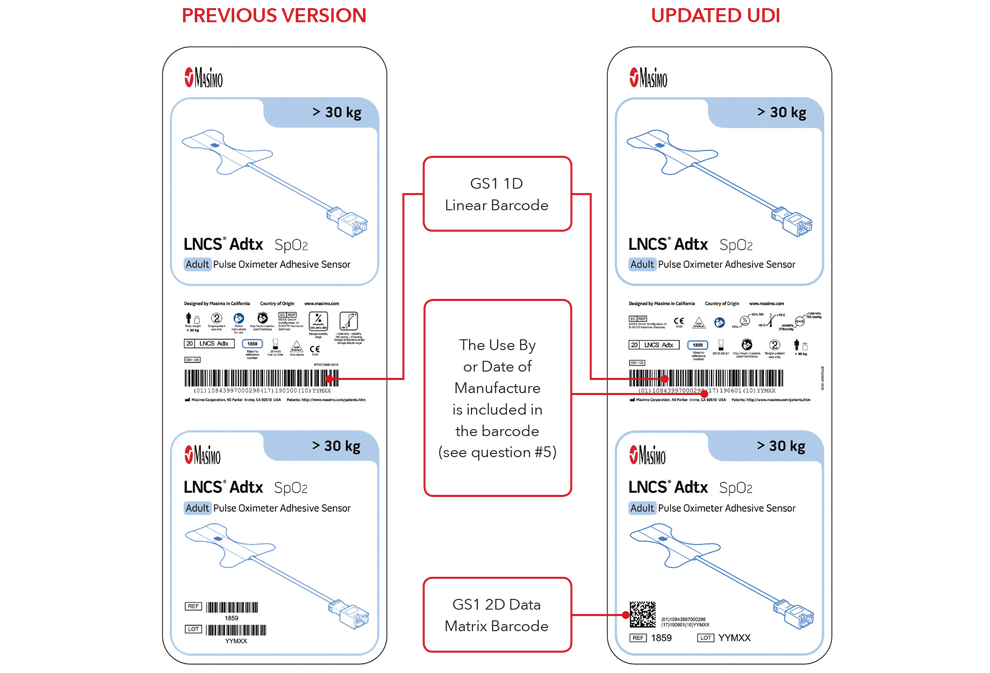
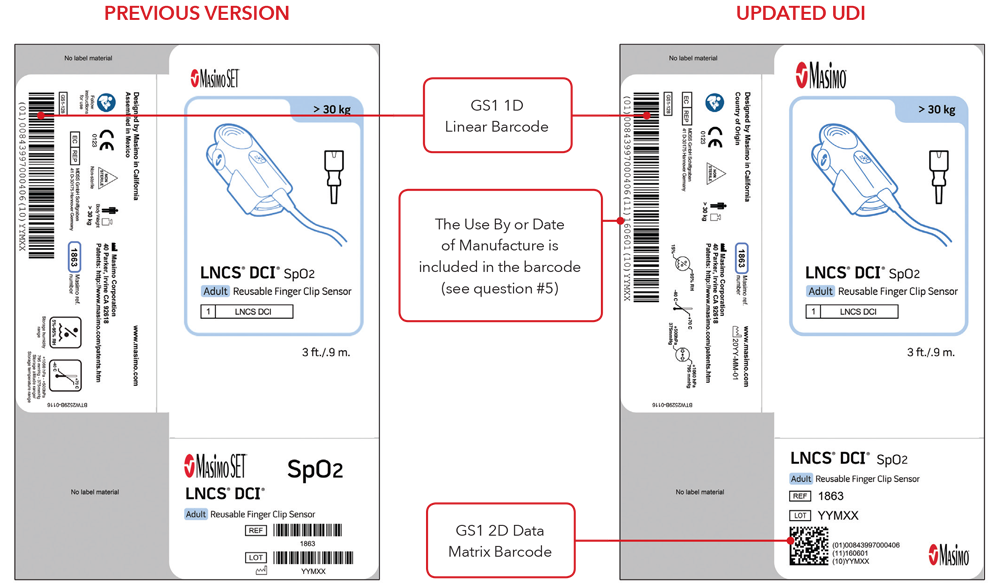
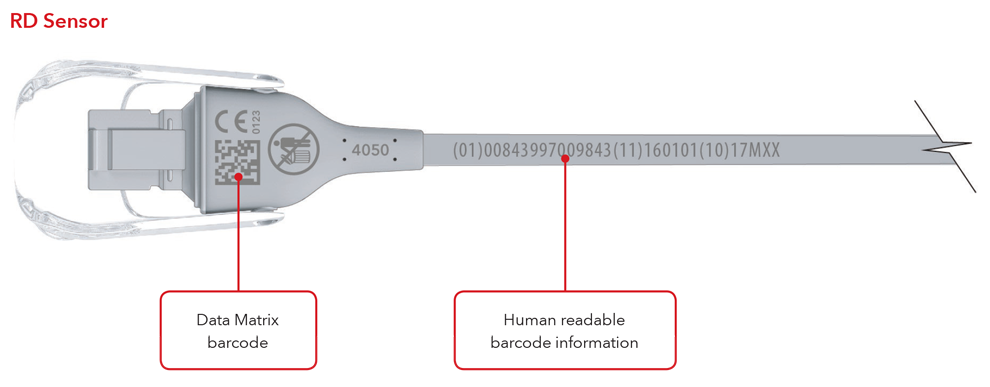
5. What will my UDI label and direct part marking look like?
Below are examples of:
- Sensor box labels with previous version and updated UDI labels
- Sensor with direct part marking
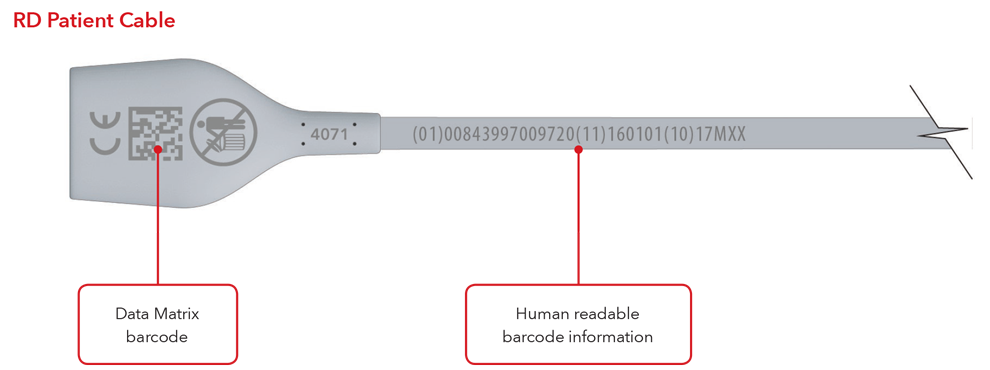
- Cable with direct part marking
Please Note: Depending on type of product, the label will contain one of two dates. For products that have a “Use By” date, it will appear as YYYY-MM-DD beneath the expiration symbol, and will also be encoded as YYMMDD preceded by the production identified (17) within the GS1 and 2D barcodes. For products that do not have a “Use By” date, the Date of Manufacture will appear as YYYY-MM-DD adjacent to the date of manufacture symbol and also be encoded as YYMMDD preceded by the production identifier (11) within the GS1 and 2D barcodes.
Sensor Box Label Examples
Sensor and Cable with Direct Part Marking Examples
6. What is a Global Trade Item Number (GTIN)?
A GTIN is a globally unique GS1 Identification Number used to identify a "trade item." GTINs are assigned by the brand owner (labeler) of the product and are used to identify products as they move through the global supply chain to the end user, such as a hospital. If you are not currently using the GS1 system with GTINs to manage your products, you will need to obtain the GTINs for the products you are purchasing.
For more information about GTINs, please contact GS1 at: https://www.gs1us.org/what-we-do/standards.
7. How Do I Get Product GTIN(s)?
Email Masimo Customer Service:
US: customerorders@masimo.com
Outside US: emeasales@masimo.com
Please include your customer ID and the product part number(s) in the email.
8. I am currently entering or scanning the Masimo LOT and/or REF barcodes on the label. Should I expect any disruptions?
Yes. The LOT or REF barcodes on the label will be removed and replaced with text and can no longer be scanned. For LOT information, you will either scan the GS1 barcode or locate it next to the LOT symbol on the label. The REF information can be identified in the corresponding GTIN. For more information on GTINs, see question #6 above.
9. Will Masimo product numbers change as UDI labeling is implemented?
No, Masimo product numbers will remain the same.
10. What is the new standardized date format and when does it go into effect?
The UDI rule adopted the standardized date format YYYY-MM-DD on device labels. Dates on labels will be in the new format no later than the date on which the label of the device must bear a UDI. For more information on the implementation timeline, see question #3 above.
11. Will Masimo be providing product information online?
The GUDID is a publicly searchable database that contains the device identification (DI) portion of the UDI for every medical device as well as other required product information. All medical device companies must submit DI information to the GUDID. Masimo has started populating the GUDID and will ensure that all products are added according to or in advance of the UDI implementation timeline.
12. What about existing inventories? Do manufacturers have to re-mark them?
No, there are two exceptions for existing inventories:
- Devices that are in commercial distribution prior to the applicable compliance date do not have to comply with the final rule.
- Devices that are manufactured and labeled prior to the applicable compliance date are also exempt from compliance. However, this exception expires 3 years after the applicable compliance date.
13. Do all devices need to be directly marked with their UDIs?
No. The rule only requires direct marking for reusable medical devices that need to be reprocessed (high level cleaning/sterilization) before reuse.
For more information on the direct marking implementation timeline, see question 3 above.
For more information on the UDI system, visit Unique Device Identification (UDI) on the FDA website:
http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/UniqueDeviceIdentification/
If you have additional questions about how Masimo is complying with UDI regulations, please contact Masimo Customer Service at:
US: customerorders@masimo.com
Outside US: emeasales@masimo.com
PLCO-001927/PLMM-10045C-0818